by Giuliana Miglierini
Rare cancers, which account for approx. 22% of new cases in Europe, represent an area of low business interest for the pharmaceutical industry, due to the limited number of patients compared to the very high costs to develop targeted treatments. It is thus important to consider the possibility for already existing medicines to be repurposed for a new indication. Lower costs of development and risk of failure, and a shorter time frame to reach registration are upon the main advantages of repurposing compared to de novo development, highlights the Policy Brief presented during the Joint meeting of EU Directors for Pharmaceutical Policy & Pharmaceutical Committee of 8 and 9 July 2021.
The experts addressed more specifically the possibility to achieve non-commercial repurposing of off-patent cancer medicines, which are commonly used off-label to treat patients not responsive to other more innovative types of therapies.
The issue of non-commercial development
The request of a new indication for an already marketed medicine has to be submitted by the Marketing authorisation holder (MAH). This greatly hampers the access to noncommercial repurposing by independent research institutions, as they would need to find an agreement with the MAH, the only responsible for all the interactions with regulatory authorities, at the central (EMA) or national level.
Considering the issue from the industrial point of view, this type of external request may prove difficult to be answered positively, when taking into consideration the very low return on investment that can be expected from a repurposed off-patent medicine. Even EU incentives schemes, such as those on data exclusivity and orphan designation, may not be sufficiently attractive for the industry. Current incentives schemes, for example, allow for an additional year of exclusivity in case of a new indication for a well-established substance, a 10-year market exclusivity
plus incentives in case of an authorised medicine granted with orphan designation, or the extension of the supplementary protection certificate for paediatric studies (plus 2 years market exclusivity for orphans).
The following table summarises the main issues and potential solutions involved in the setting of a specific reference framework for the repurposing of off-patent medicines for cancer, as reported in the WHO’s Policy Brief.
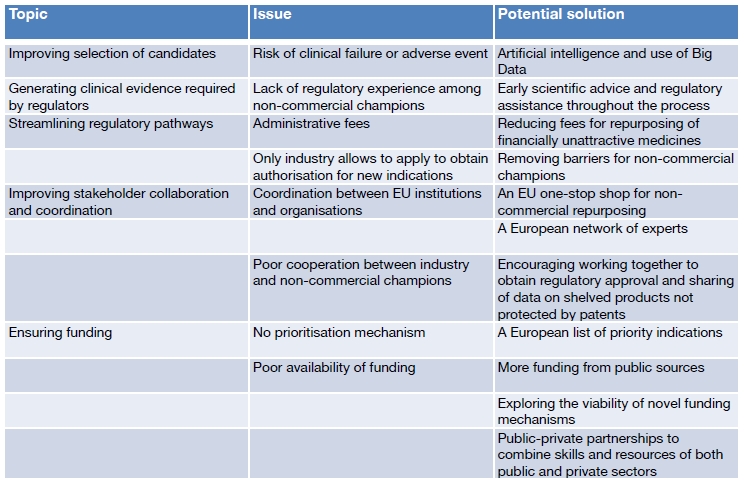
(Source: Repurposing of medicines – the underrated champion of sustainable innovation. Copenhagen: WHO Regional Office for Europe; 2021. Licence: CC BY-NC-SA 3.0 IGO)
Many projects active in the EU
The European Commission started looking at the repurposing of medicines with the 2015-2019 project Safe and Timely Access to Medicines for Patients (STAMP). A follow-up phase of this initiative should see the activation in 2021 of a pilot project integrated with the new European Pharmaceutical Strategy.
Several other projects were also funded in the EU, e.g. to better train the academia in Regulatory Science (CSA STARS), use in silico-based approaches to improve the efficacy and precision of drug repurposing (REPO TRIAL) or testing the repurposing of already marketed drugs (e.g. saracatinib to prevent the rare disease fibrodysplasia ossificans progressive, FOP). A specific action aimed to build a European platform for the repurposing of medicines is also included in Horizon Europe’s Work programme 2021 –2022; furthermore, both the EU’s Beating Cancer Plan and the Pharmaceutical Strategy include actions to support non-commercial development for the repurposing of medicines.
According to the WHO’s Policy Brief, a one-stop shop mechanism could be established in order for selected non-commercial actors, the so-called “Champions”, to act as the coordination point for EU institutions involved in the funding of research activities aimed to repurposing. This action may be complemented by the support to public–private partnerships involving research, registration and manufacturing and targeted to guarantee volumes for non-profitable compounds.
Among possible non-profit institutions to access funding for repurposing research in cancer are the European Organisation for Research on Cancer (EORTC) and the Breast Cancer International Group. An overview of other existing initiatives on repurposing has been offered during the debate by the WHO’s representative, Sarah Garner.
How to address repurposing
Looking for a new indication is just one of the possible points of view from which to look at the repurposing of a medicine. Other possibilities include the development of a new administration route for the same indication, the setup of a combination form instead of the use of separated medicinal products, or the realisation of a drug-medical device combination.
A change of strategy in the war on cancer may be useful, according to Lydie Meheus, Managing Director of the AntiCancer Fund (ACF), and Ciska Verbaanderd.
Keeping cancer development under control may bring more efficacy to the intervention than trying to cure it, said ACF’s representatives. The possible approaches include a hard repurposing, with a medicine being transferred to a completely new therapeutic area on the basis of considerations about the tumor biology and the immunological, metabolic and inflammatory pathways, or a soft repurposing within the oncology field, simply looking to new indications for rare cancers.
From the regulatory point of view, a possible example for EMA on how to address the inclusion of new off-label uses of marketed medicines is given by the FDA, which may request a labeling change when aware of new information beyond the safety ones.
The Champion framework
The Champion framework, proposed as a result of the STAMP project, is intended to facilitate data generation and gathering compliant to regulatory requirements for a new therapeutic use for an authorised active substance or medicine already free from of intellectual property and regulatory protection.
A Champion is typically a not-for-profit organisation, which interacts with the MAH in order to include on-label what was previously off-label, using existing regulatory tools (e.g innovation offices and scientific and/or regulatory advice). The Champion shall coordinate research activities up to full industry engagement and would be responsible for filing the initial request for scientific/regulatory advice on the basis of the available data. The pilot project to be activated to test the framework will be monitored by the Repurposing observatory group (RepOG), which will report to the Pharmaceutical Committee and will issue recommendations on how to deal with these types of procedures.
AI to optimise the chances of success
Artificial intelligence (AI) may play a central role in the identification of suitable medicines to be repurposed for a target indication, as it supports the collection and systematic analysis of very large amounts of data. The process has been used during the Covid pandemic, for example, when five supercomputers analysed more than 6 thousand molecules and identified 40 candidates for repurposing against the viral infection.
AI can be used along drug development process, making it easier to analyse the often complex and interconnected interactions which are at the basis of the observed pharmacological effect (e.g drug-target, protein-protein, drug-drug, drug-disease), explained Prof. Marinka Zitnik, Harvard Medical School.
To this instance, graphic neural networks can be used to identify a drug useful to treat a disease, as it is close to the disease in “pharmacological space”. The analysis may also take into account the possible interactions with other medicines. This is important to better evaluate the possible side effects resulting from co-prescribing; annual costs in treating side effects exceed $177 billion in the US alone, according to Prof. Zitnik.